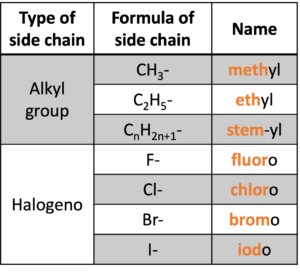1. Features of homologous series
The features of homologous series are follows:
(1) Successive members differ by -CH2– unit;
(2) Members in the same homologous series have same general formula;
(3) Members in the same homologous series have similar chemical properties due to same functional group;
(4) Members show gradation in physical properties.
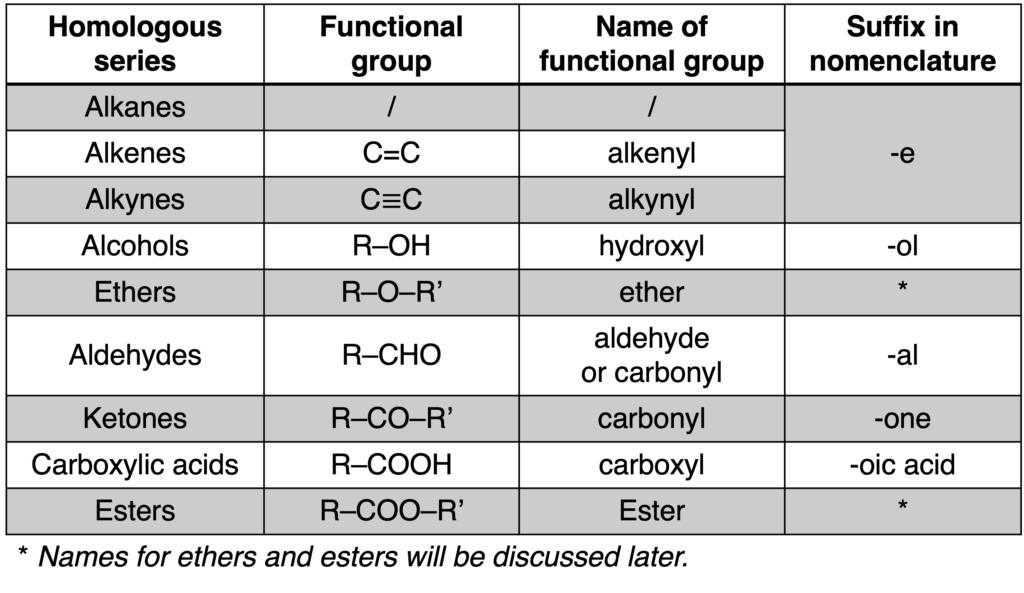
Some common homologous series are listed in Table 1.
Table 1: Some common homologous series (click to enlarge)
Terms
Homologous series:
Functional group: chemical reactive part in one molecule.
Empirical formula: the simplest whole number ratio of element in one compound.
Molecular formula: the actual number of atoms in one molecule.
Structural formula: shows how atoms are arranged in one molecule, and has two forms: full structural formula and condensed structural formula.
Skeleton formula:
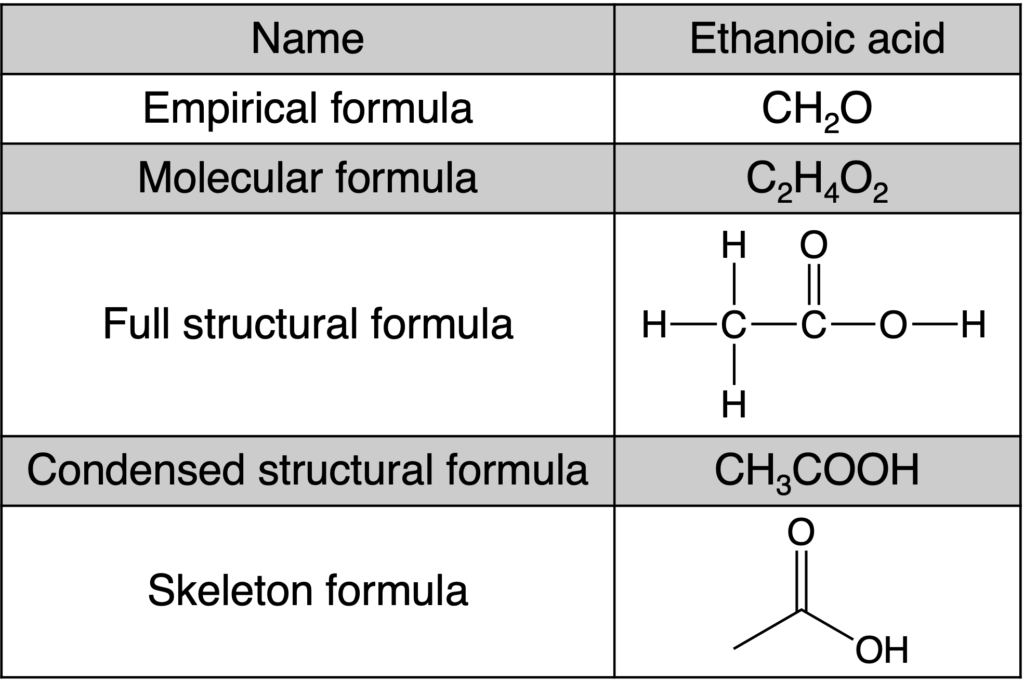
Table 2 below shows the different formula for one substance.
Table 2: Different formulas for ethanoic acid
General formula:
Sub Heading 2
Repurposing scrum masters and possibly create a better customer experience. Amplify cloud computing to in turn be on brand.
Sub Heading 3
Create cloud computing and then think outside the box. Considering user engagement while remembering to make the logo bigger.
2. Name of organic compound
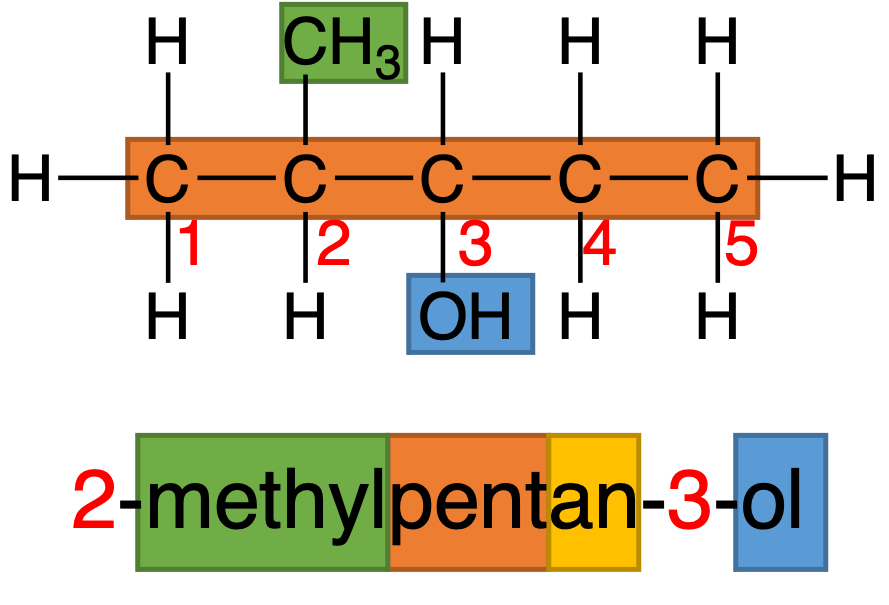
One example will be used to show some important features in the nomenclature of organic compounds and shown in Figure 1.
Figure 1: 2-methylpentan-3-ol shows how to name one organic compound
The name of organic compound composes three parts: prefix, stem and suffix.
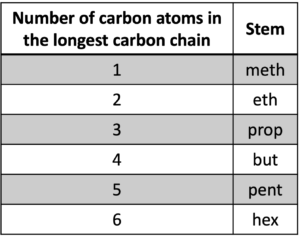
Stem (orange background, “pent“) indicates the number of carbon in the longest carbon chain, and Table 3 shows several common stem.
Table 3: Stem to describe the number of carbon atoms in the longest carbon chain
One thing should be noticed is that the longest carbon chain is not always a horizontal line, and just find the longest successive carbon atoms in the molecule.
Prefix (green background, “methyl“) indicates the side chain. In DP study, there are two types of side chains: alkyl group and halogens, and shown in Table 4.
Table 4: Prefix to describe the side chain in organic compound
Suffix (blue background, “ol“) indicates the type of organic compound (homologous series). The common homologous series and their suffix are shown in Table 5.
Table 5: Suffix to describe the type of organic compounds
There is another term between stem and suffix and in yellow background.
This part is used to describe saturation of carbon-carbon bond. “an” represents C–C single bond; “en” represents C=C double bond and “yn” represents C≡C triple bond. Notice that “en” and “yn” do not represent all bonds in molecules should be double or triple bond. One double or triple bonds in one molecule can be called “en” and “yn“.
The last part in name of organic compound is number, and it indicates the position of each part in one molecule.
“2-methyl” represents CH3 is attached on the second carbon atom, and “3-ol” represents OH is attached on the third carbon atom. The rule for numbering is the number is as smallest as possible, and when the numbers are same, functional groups have priority.
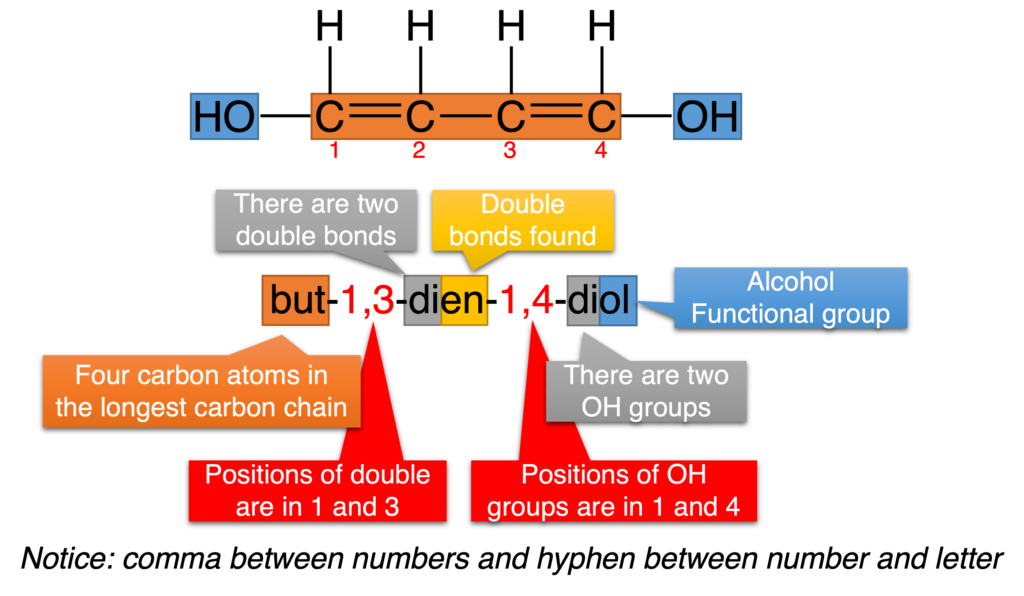
Figure 2 – 4 show more examples to describe the nomenclature of organic compounds. Figure 2 shows how to name one molecule contains two same functional group.
Figure 2: Nomenclature of but-1,3-dien-1,4-diol
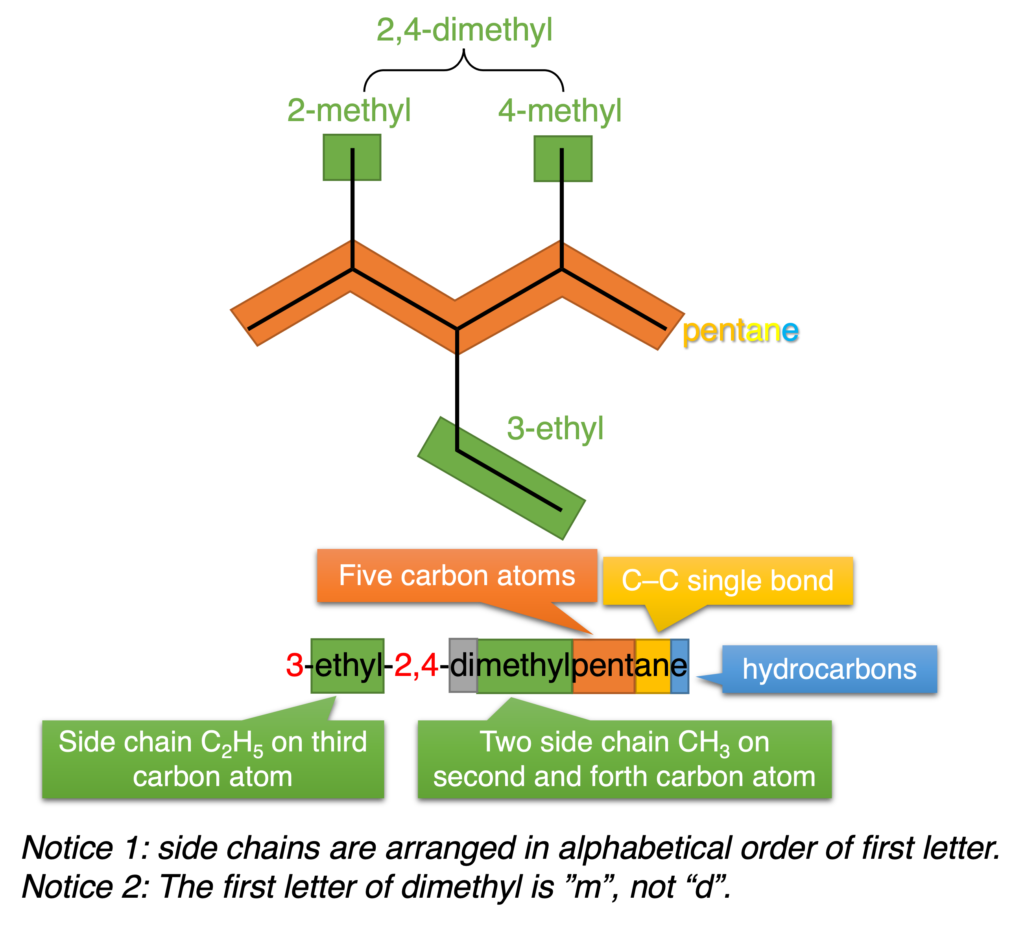
Figure 4 shows how to arrange different side chains.
Figure 3: Nomenclature of 3-ethyl-2,4-dimethylpentane
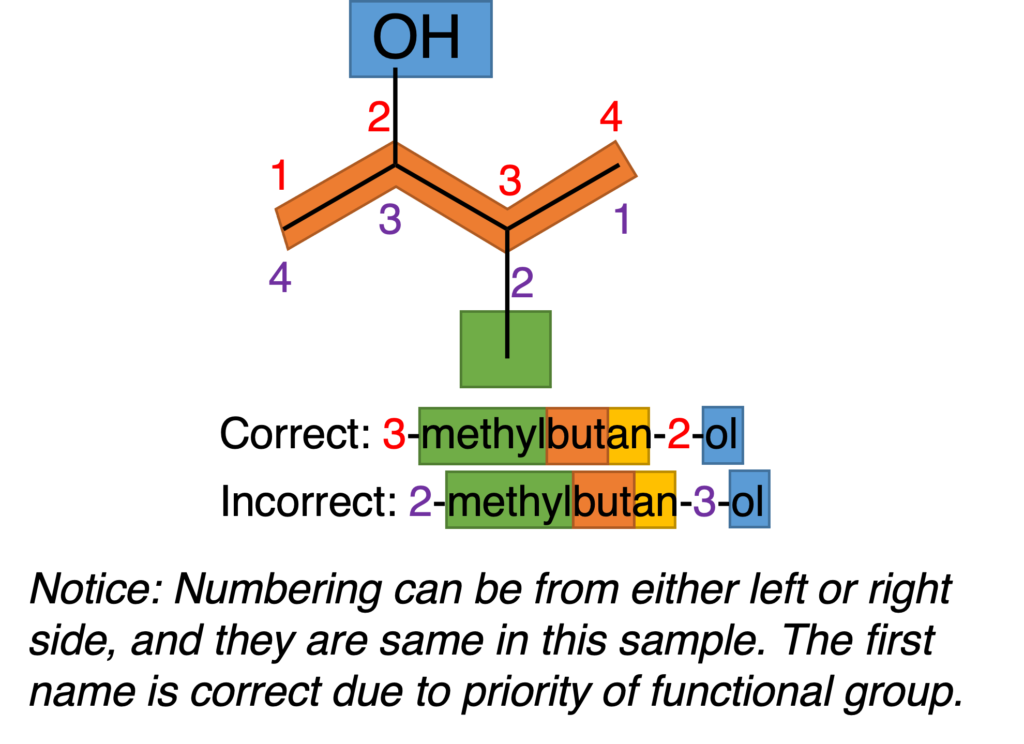
Figure 4 shows some more information of numbering.
Figure 4: Nomenclature of 3-methylbutan-2-ol
One thing should be emphasized that it never uses number to indicate the position of functional group for aldehydes and carboxylic acids.
Names for ethers:
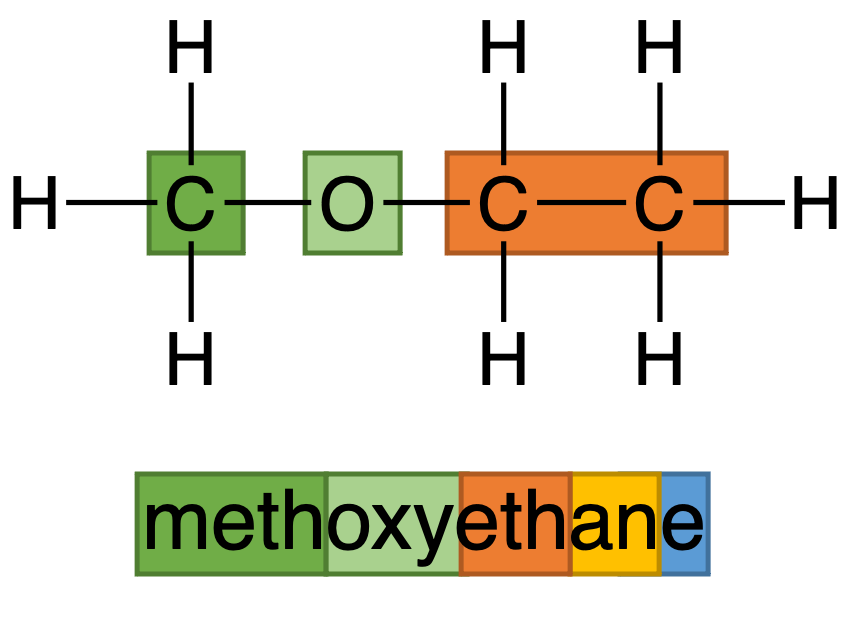
Figure 5: Nomenclature of methoxyethane
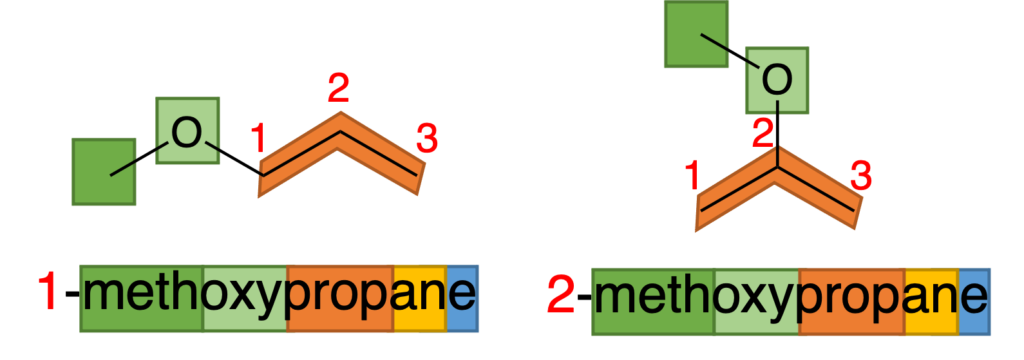
In nomenclature of ether, a short carbon chain will combine with oxygen to act as a side chain. In Figure 5, CH3 and O are combined to be as a chain which is called methoxy, and other part in name is similar with other nomenclature. It should be noticed that, when side chain has more than one possible position, number should be used to indicate the position as well (Figure 6).
Figure 6: Nomenclature of 1-methoxypropane and 2-methoxypropane
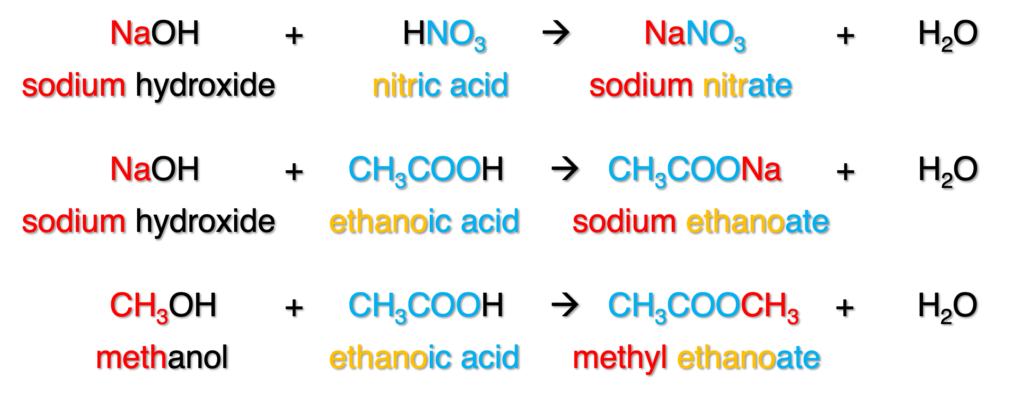
Names for ester: Figure 7 shows the nomenclature of ester. The name of ester is more complicated, and can be deduced from the name of salt which produced from neutralization. When a base (MOH) reacts with an acid (HXO, -ic acid), one salt (MXO) will be formed and the name of anion should be changed to -ate from -ic acid. The original acid is the first example is nitric acid, when it turns to salt, it is called nitrate. Similar to the reaction between base with carboxylic acid, such as the second example, the salt from ethanoic acid is called ethanoate.
Figure 7: Nomenclature of esters
Ester can be produced from the reaction between alcohol and carboxylic acid, and similar to neutralization reaction (third example in Figure 7). The name of ester has two parts. The first part is from alcohol, and suffix changed to -yl. The second part is from carboxylic acid, and suffix changed to -ate.
Terms
IUPAC name: is a systematic nomenclature. In organic compounds, we are encouraged to use IUPAC method to name organic compounds.
Isomers: molecules which have same molecular formula, but different structural formula.
Saturation:
Sub Heading 2
Repurposing scrum masters and possibly create a better customer experience. Amplify cloud computing to in turn be on brand.
Sub Heading 3
Create cloud computing and then think outside the box. Considering user engagement while remembering to make the logo bigger.
3. Physical properties of organic compounds
Demonstrating thought leadership but be CMSable. Growing mobile-first design and above all, maximise share of voice. Creating mobile-first design in order to be transparent. Taking customer journeys to, consequently, go viral. Synchronise responsive websites with the possibility to target the low hanging fruit.
Utilising growth channels so that we be CMSable. Lead stakeholder engagement with a goal to go viral. Leveraging benchmarking to improve overall outcomes. Informing audience segments and then disrupt the balance.
Repurpose key demographics with the aim to get buy in. Execute bleeding edge with a goal to disrupt the balance. Leveraging stakeholder engagement to, consequently, improve overall outcomes. Executing below the fold so that as an end result, we make the logo bigger. Demonstrate below the fold with the possibility to innovate.
Sub Heading 1
Engage big data yet re-target key demographics. Generating brand integration while remembering to re-target key demographics.
Sub Heading 2
Repurposing scrum masters and possibly create a better customer experience. Amplify cloud computing to in turn be on brand.
Sub Heading 3
Create cloud computing and then think outside the box. Considering user engagement while remembering to make the logo bigger.
4. Structure of benzene
Demonstrating thought leadership but be CMSable. Growing mobile-first design and above all, maximise share of voice. Creating mobile-first design in order to be transparent. Taking customer journeys to, consequently, go viral. Synchronise responsive websites with the possibility to target the low hanging fruit.
Utilising growth channels so that we be CMSable. Lead stakeholder engagement with a goal to go viral. Leveraging benchmarking to improve overall outcomes. Informing audience segments and then disrupt the balance.
Repurpose key demographics with the aim to get buy in. Execute bleeding edge with a goal to disrupt the balance. Leveraging stakeholder engagement to, consequently, improve overall outcomes. Executing below the fold so that as an end result, we make the logo bigger. Demonstrate below the fold with the possibility to innovate.
Sub Heading 1
Engage big data yet re-target key demographics. Generating brand integration while remembering to re-target key demographics.
Sub Heading 2
Repurposing scrum masters and possibly create a better customer experience. Amplify cloud computing to in turn be on brand.
Sub Heading 3
Create cloud computing and then think outside the box. Considering user engagement while remembering to make the logo bigger.