1. Formation and definition of ionic bonding
Ionic bonding was formed from electron transfer, and shown in Figure 1.
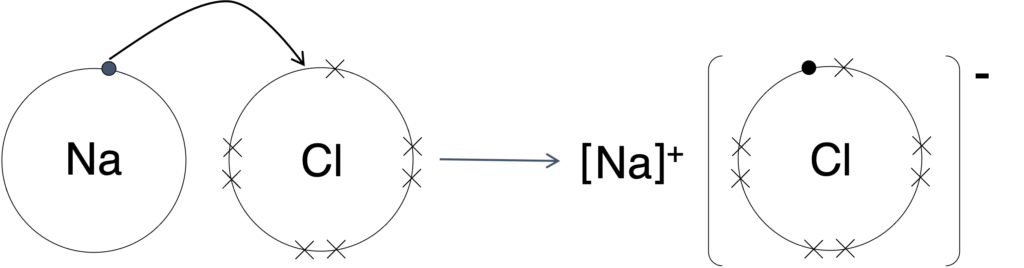
Figure 1: Formation of ionic bonding
Sodium atom has one valence electron. For achieving octet configuration, sodium atom will lose one electron to form cation, Na+.
Chlorine atom has seven valence electrons. For achieving octet configuration, chlorine atom will lose one electron to form anion, Cl–.
Attraction will be formed between cation and anion, and it is called ionic bonding, and formula is NaCl.
Terms
Ionic bonding: electrostatic attraction between cation and anion.
Sub Heading 2
Repurposing scrum masters and possibly create a better customer experience. Amplify cloud computing to in turn be on brand.
Sub Heading 3
Create cloud computing and then think outside the box. Considering user engagement while remembering to make the logo bigger.
2. Formula and name of ionic compound
Formula for ionic compounds
Principle for writing formula of ionic compound is zero total charge of cation and anion. Some charge for monatomic anions can be deduced from periodic table. For example:
Metals in group 1 have one valence electron, and the charge on ion should be 1+. Such as Li+, Na+ and K+.
Metals in group 2 have two valence electrons, and the charge on ion should be 2+. Such as Mg2+, Ca2+ and Ba2+.
Metals in group 13 have three valence electrons, and the charge on ion should be 3+. Such as Al3+.
Nonmetals in group 15 have five valence electrons, and the charge on ion should be 3-. Such as N3-, P3-.
Nonmetals in group 16 have five valence electrons, and the charge on ion should be 2-. Such as O2-, S2-.
Nonmetals in group 15 have five valence electrons, and the charge on ion should be 1-. Such as F–, Cl–, Br–, I–.
Some polyatomic ions should be memorized. And common ions are listed in Table 1.

Table 1: Names and formulae for some common polyatomic ions.
The method to deduce the formula of ionic compound is called “cross-method” and show in Figure 2.
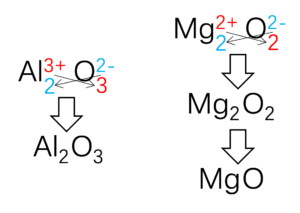
Figure 2: Deduce the formula of ionic compound
It should be noticed that formula for ionic compound is empirical formula, so the formula should keep the simplest whole number ratio. For magnesium oxide, it is not Mg2O2 and should be simplified into MgO.
Name for ionic compounds
Some names of polyatomic ion are listed in Table 1. For monatomic ions, there are two different cases depend on the charge on ion.
Metal cation with fixed charge:
1+: Group 1 alkali metals (Na+, K+, etc.), and Ag+;
2+: Group 2 alkaline earth metals (Mg2+, Ca2+, etc.), and Zn2+;
3+: Al3+;
Names of ions which have fixed charge is same to the name of element, but followed by ion. For example, Ca2+ is called calcium ion.
Metal cation with varied charge: most metal can lose varied number of electrons to form cation with varied charge. So the names for these cations should use Roman numerals to indicate the charge on them. For example, Fe2+ and Fe3+ are called iron (II) and iron (III) ion, respectively.
Nonmetal anions have fixed charge on ion and can be predicted from periodic table. The names for them are “stem of element” and followed by “-ide” as suffix. Table 2 lists name for common nonmetal anions.
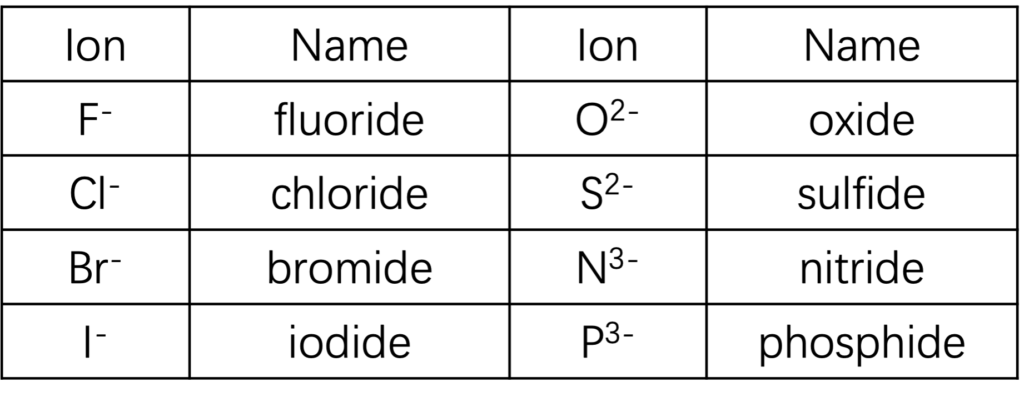
Table 2: Name for monatomic anions
The general rule for name of ionic compound is from cation’s name to anion’s name.
For example:
NaCl is composed by Na+ (sodium ion) and Cl- (chloride ion), and name for NaCl is sodium chloride.
CuSO4 is composed by Cu2+(copper (II) ion) and SO42-(sulfate), and name for CuSO4 is copper(II) sulfate.
Terms
Ions: charged particles, and can be formed from loss or gain of electrons.
Cation: positively charged ion, and can be produced from loss of electrons.
Anion: negatively charged ion, and can be produced from gain of electrons.
Monatomic ion: ion which contains one atom.
Polyatomic ion: ion which contains more than one atom covalently bonded together.
Sub Heading 2
Repurposing scrum masters and possibly create a better customer experience. Amplify cloud computing to in turn be on brand.
Sub Heading 3
Create cloud computing and then think outside the box. Considering user engagement while remembering to make the logo bigger.
3. Ionic lattice structure
Bonding is the attraction between oppositely charged particles, but structure is used to describe how a lot of particles arranged, and lattice structure is the arrangement of particles in solid state.
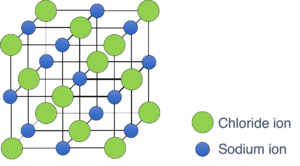
Different substance has different structure, and you are required to understand the structure of NaCl and shown in Figure 2.
Figure 2: The lattice structure of sodium chloride
Some key words can be used to describe the lattice structure of sodium chloride.
(1) Giant
(2) Cubic
(3) 6: each chloride is surrounded by six sodium ion, and each sodium ion is surrounded by six chloride.
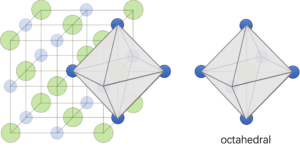
(4) Octahedral: six particles are surrounded central ion to form the shape which is called octahedral and shown in Figure 3.
Figure 3: Octahedral was shown by six sodium ions surrounded chloride ion
Sub Heading 1
Engage big data yet re-target key demographics. Generating brand integration while remembering to re-target key demographics.
Sub Heading 2
Repurposing scrum masters and possibly create a better customer experience. Amplify cloud computing to in turn be on brand.
Sub Heading 3
Create cloud computing and then think outside the box. Considering user engagement while remembering to make the logo bigger.
4. Physical properties of ionic compounds
(1) High melting point and boiling point: because of strong ionic bonding.
Additional knowledge: grater charge on ion and smaller ionic radius, stronger ionic bonding and higher melting point.
(2) Brittle
Figure 4: Diagram to show the brittleness of ionic compound
As shown in Figure 4, when the external force is applied on the lattice structure, the layers will slide. Same charged ions will be neighbored and repel each other to break the structure.
(3) Electrical conductivity
In solid state, ionic compound cannot conduct electricity because all ions are fixed on lattice.
In molten state and in aqueous, ionic compound can conduct electricity due to free-moving ions.
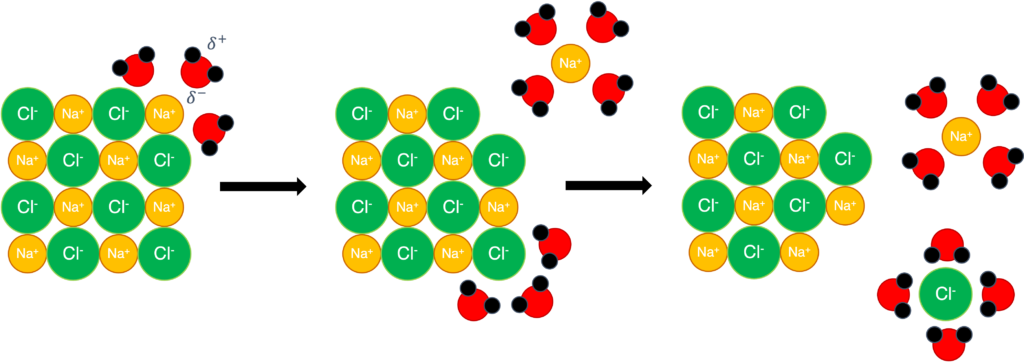
(4) Solubility in water: soluble in water
Figure 5: Dissolving process of sodium chloride in water
Figure 5 shows the dissolving process when sodium chloride in water. Positively and negatively charged ion will be dispersed into water because water molecule is polar molecule and can for ion-dipole interaction.
Sub Heading 1
Engage big data yet re-target key demographics. Generating brand integration while remembering to re-target key demographics.
Sub Heading 2
Repurposing scrum masters and possibly create a better customer experience. Amplify cloud computing to in turn be on brand.
Sub Heading 3
Create cloud computing and then think outside the box. Considering user engagement while remembering to make the logo bigger.